Large-scale next-generation sequencing (NGS) studies have suggested common patterns of co-occurrence or mutual exclusivity between genetic alterations in chronic lymphocytic leukemia (CLL). However, little is known about how most of these alterations cooperate to drive CLL pathogenesis, as well as the impact of these concurrencies in clinical outcome. In this regard, we investigated the clinical and biological impact of the co-occurrence of high-risk lesions such as del(11q)/ATM mutation and del(17p)/TP53 mutation by integrating NGS and CRISPR/Cas9 approaches.
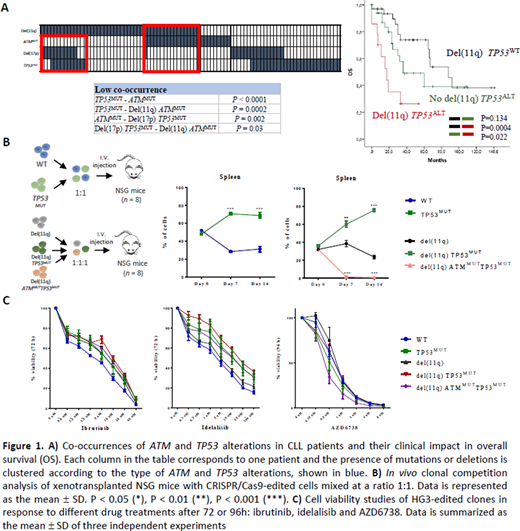
To address these questions, we first analyzed the mutational profile of 271 CLLs (17.3% del(11q); 10.7% del(17p)). The most frequently mutated genes were NOTCH1 (20%), TP53 (14%), SF3B1 (11%) and ATM (10%). Within del(11q), 32% showed TP53 alterations (53% biallelic; 47% monoallelic). Interestingly, patients harboring combined del(11q) and TP53 alterations by either mutation or deletion (del(11q) TP53ALT) exhibited significantly shorter overall survival (OS) than del(11q) CLLs without TP53 alterations (del(11q) TP53WT) and those TP53 altered without del(11q) (no del(11q) TP53ALT) (median 17 vs. 88, 36 months; P=0.0004, P=0.02). Conversely, we observed a significant lack of ATM mutations in CLLs with biallelic TP53 alterations (P=0.002) and a mutual exclusivity between biallelic TP53 and biallelic ATM losses (P=0.03)(Fig 1A).
Based on the NGS results, we next used the CRISPR/Cas9 system to model monoallelic and biallelic ATM and TP53 loss in vitro. We generated isogenic HG3-Cas9 CLL-derived cell lines harboring monoallelic del(11q) (targeting 11q22.1/11q23.3 regions) and further loss-of-function mutations in ATM and/or TP53 to mimic all the possible combinations observed in our CLL cohort. By proliferation assays, we noted that the introduction of TP53 mutations increased the proliferation rates in both HG3WT and HG3-del(11q) cells. In contrast, the introduction of an ATM truncating mutation on the remaining allele of the HG3-del(11q) TP53MUT clone, suppressed this proliferative advantage, with growth rates comparable to those of HG3-del(11q). Accordingly, DNA content analysis by propidium iodide revealed that cells harboring biallelic ATM and TP53 loss also showed mitotic and cell cycle defects.
To further evaluate the implications of these alterations in the clonal dynamics of CLL in vivo, we performed fluorescence-based clonal competition experiments by injecting these edited cell lines intravenously into NGS mice. First, we observed that HG3-TP53MUT cells outgrew HG3WT cells in spleen of xenotransplanted mice 14 days after injection (P<0.001). In a second experiment, HG3-del(11q), HG3-del(11q) TP53MUT and HG3-del(11q) ATMMUTTP53MUT cells were injected. Strikingly, HG3-del(11q) TP53MUT cells were able to outcompete HG3-del(11q) cells in spleen and bone marrow (P<0.001; P<0.001). By contrast, HG3-del(11q) ATMMUTTP53MUT cells failed to engraft neither in spleen nor bone marrow, being outcompeted by the other injected cells (P<0.001), providing biological insights on the mutual exclusivity of these genetic events in CLL (Fig 1B).
We next assessed whether these cell models could predict responses of these combined abnormalities to BTK and PI3K inhibitors. We observed that HG3-del(11q) TP53MUT and HG3-TP53MUT cells showed partial response to ibrutinib and idelalisib, although the IC50 values were still higher than the ones observed in HG3WT clones, especially with idelalisib (27.4 and 20.6 vs. 1.8 uM, respectively). Nonetheless, we found that HG3-del(11q) TP53MUT cells were highly sensitive to novel preclinical drugs that have been shown to be effective in TP53 deficient cells such ATR inhibitors, with an IC50 value comparable to HG3WT cells (mean IC50 0.55 vs. 0.67 uM) (Fig 1C).
In summary, we show that mutations in TP53 can appear in a subset of monoallelic del(11q) CLL cases, conferring a synergistic clonal advantage in vivo, and therefore a dismal clinical impact on the OS of this CLL subgroup. In addition, the biological basis of mutual exclusivity of biallelic ATM and TP53 alterations in CLL was assessed, underscoring the importance of the number of alleles affected by these alterations, and establishing novel pre-clinical models for the study of the biology and therapeutic response of concurrent genetic abnormalities in the disease.
Funding: PI18/01500 FI19/00191 CD19/00222
*MQA CPC equal contr
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal